Artificial Intelligence/ Computational Biology Innovation Lab Launched by the Mega Companies AstraZeneca, Merck, Pfizer, Teva, AWS, IBF – News & Updates
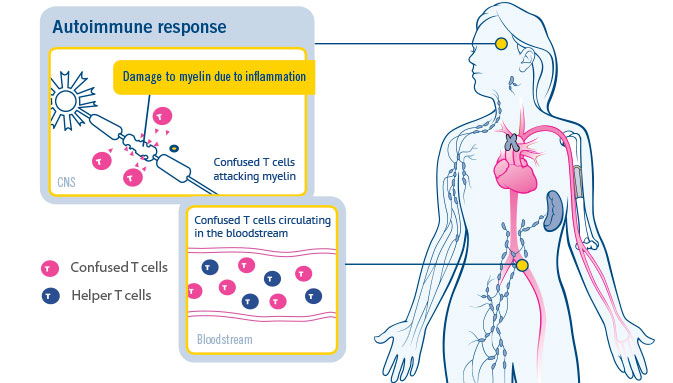
TEVA ANNOUNCES SUCCESSFUL RESULTS OF PHASE III STUDY WITH ORAL LAQUINIMOD FOR MULTIPLE SCLEROSIS • Laquinimod study met prima

Active Biotech and Teva together in a Phase II clinical trial against Huntington's disease - Labiotech.eu